Latent Heat Of Vaporization
In general, when a material changes phase from solid to liquid, or from liquid to gas a certain amount of energy is involved in this change of phase. In case of liquid to gas phase change, this amount of energy is known as the enthalpy of vaporization, also known as the heat of vaporization or heat of evaporation. As an example, see the figure, which descibes phase transitions of water.
Latent heat is the amount of heat added to or removed from a substance to produce a change in phase. This energy breaks down the intermolecular attractive forces, and also must provide the energy necessary to expand the gas . When latent heat is added, no temperature change occurs. The enthalpy of vaporization is a function of the pressure at which that transformation takes place.
The temperature at which vaporization starts to occur for a given pressure is also known as the saturation temperature and at this conditions a mixture of vapor and liquid can exist together. The liquid can be said to be saturated with thermal energy. Any addition of thermal energy results in a phase transition. At the boiling point the two phases of a substance, liquid and vapor, have identical free energies and therefore are equally likely to exist. Below the boiling point, the liquid is the more stable state of the two, whereas above the gaseous form is preferred.
Melting Point Drop Of Manganese
Electrons configuration of manganese is 3d5 4s2. That electrons configuration has some stability because all five d orbits are half filled So contribution of electrons to the metallic lattice is limited in manganese. Therefore lattice is not much strong. That is the reason why manganese has a sudden drop in melting point.
Strange Isotopes: Scientists Explain A Methane Isotope Paradox Of The Seafloor
Why methane carbon isotopes in the deep sea behave so differently than expected
Max Planck Institute for Marine Microbiology
image: The Guaymas Basin hydrothermal vents – the & quot home& quot of the studied methane-oxidizing microorganisms. The heat loving microorganisms thrive under the orange microbial mat in the background. The high temperatures of the rising waters blur parts of the image.view more
Methane, a chemical compound with the molecular formula CH4, is not only a powerful greenhouse gas, but also an important energy source. It heats our homes, and even seafloor microbes make a living of it. The microbes use a process called anaerobic oxidation of methane , which happens commonly in the seafloor in so-called sulfate-methane transition zones – layers in the seafloor where sulfate from the seawater meets methane from the deeper sediment. Here, specialized microorganisms, the ANaerobically MEthane-oxidizing archaea, consume the methane. They live in close association with bacteria, which use electrons released during methane oxidation for sulfate reduction. For this purpose, these organisms form characteristic consortia.
Isotopes reveal reaction pathways
Nature doesn’t follow the textbook: Light methane in sulfate-methane transition zones
The availability of sulfate governs the isotopes effects in AOM
Recommended Reading: How To Make Your Belly Stop Hurting On Your Period
Atomic Number Of Helium
Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e equals to 1,602 x 10-19 coulombs. In a neutral atom there are as many electrons as protons moving about nucleus. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements.
See also: Atomic Number Does it conserve in a nuclear reaction?
Coefficient Of Thermal Expansion Of Helium
Linear thermal expansion coefficient of Helium is µm/
Thermal expansion is generally the tendency of matter to change its dimensions in response to a change in temperature. It is usually expressed as a fractional change in length or volume per unit temperature change. Thermal expansion is common for solids, liquids and for gases. Unlike gases or liquids, solid materials tend to keep their shape when undergoing thermal expansion. A linear expansion coefficient is usually employed in describing the expansion of a solid, while a volume expansion coefficient is more useful for a liquid or a gas.
The linear thermal expansion coefficient is defined as:
where L is a particular length measurement and dL/dT is the rate of change of that linear dimension per unit change in temperature.
The volumetric thermal expansion coefficient is the most basic thermal expansion coefficient, and the most relevant for fluids. In general, substances expand or contract when their temperature changes, with expansion or contraction occurring in all directions.
The volumetric thermal expansion coefficient is defined as:
where L is the volume of the material and dV/dT is the rate of change of that volume per unit change in temperature.
Also Check: How To Help Bad Period Cramps
Methane Slip From Gas Engines
The use of natural gas and biogas in ICE for such applications as electricity production / cogeneration / CHP and heavy vehicles or marine vessels such as LNG carriers using the boil off gas for propulsion, emits a certain percentage of UHC, unburned hydrocarbon of which 85% is methane. The climate issues of using gas to fuel ICE may offset or even cancel out the advantages of less CO2 and particle emissions is described in this 2016 EU Issue Paper on methane slip from marine engines: “Emissions of unburnt methane were around 7 g per kg LNG at higher engine loads, rising to 2336 g at lower loads. This increase could be due to slow combustion at lower temperatures, which allows small quantities of gas to avoid the combustion process”. Road vehicles run more on low load than marine engines causing relatively higher methane slip.
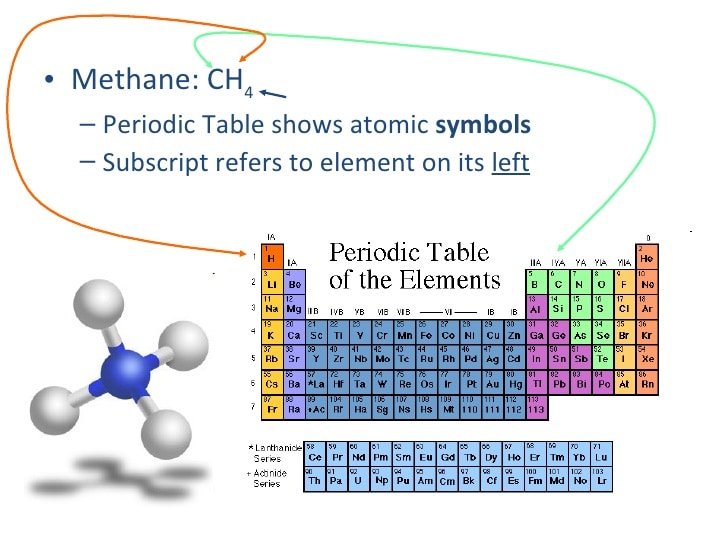
Percentage Composition Of Methane
Activity Overview
Participants analyze the atomic percentage composition of methane using the calculator, lists and graphs. The spatial orientation of the carbon and hydrogen atoms in the methane molecule is also analyzed.
Before the Activity
A review of the periodic chart might be nice.
During the Activity
Only the word download is necessary for this activity since it is a construction.
1995-2021 Texas Instruments Incorporated. All rights reserved.
Also Check: What Can Make Your Period Stop
Why Boiling Point Of Calcium Is Greater Than Potassium
Lattice strength of calcium is greater than potassium due to two reasons.
Due to these two reasons, metallic lattice of calcium is much greater than potassium.
Atomic Radius Of Helium
The atomic radius of Helium atom is 28pm .
It must be noted, atoms lack a well-defined outer boundary. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Therefore, there are various non-equivalent definitions of atomic radius.
- Van der Waals radius. In principle, Vana der Waals radius is half the minimum distance between the nuclei of two atoms of the element that are not bound to the same molecule.
- Ionic radius. An ionic radius is one-half the distance between the nuclei of two ions in an ionic bond.
- Covalent radius. Covalent radius is the nominal radius of the atoms of an element when covalently bound to other atoms.
- Metallic radius. A metallic radius is one-half the distance between the nuclei of two adjacent atoms in a crystalline structure, when joined to other atoms by metallic bonds.
Also Check: What Drinks Help With Period Cramps
Melting And Boiling Points Of Organic Compounds
Thousands of organic compounds are discovered so far by scientists in the world. With discovering lot of compounds,organic chemistry was born. In this chapter we are going to discuss melting and boiling points of organic compounds.
Boiling points of ethane and ethanoic acid is listed below and they are two different types of organic compounds.
- Methane : -161.50C
Following facts are important when we studying melting and boiling point values of organic compounds.
- Relative Molecular mass
- Ability of making hydrogen bonds
- Structure of carbon chain
Alcohols Aldehyde Ketone And Carboxylic Acids
- All of alcohols and carboxylic acids can form hydrogen bonds.
- Carboxylic acids forms most strong and highest number of hydrogen bonds among them.
- So, carboxylic acids has highest melting and boiling points.
- Dipole dipole interactions between aldehydes and ketones molecules are less strong than hydrogen bonds in alcohols.
- Therefore melting and boiling points of alcohols are higher than aldehydes and ketones.
You May Like: What Does Global Period Mean In Medical Billing
Chemical Properties Of Methane
Methane is lighter than air, having a specific gravity of 0.554. It is only slightly soluble in water. It burns readily in air, forming carbon dioxide and water vapour the flame is pale, slightly luminous, and very hot. The boiling point of methane is 162 °C and the melting point is 182.5 °C . Methane in general is very stable, but mixtures of methane and air, with the methane content between 5 and 14 percent by volume, are explosive. Explosions of such mixtures have been frequent in coal mines and collieries and have been the cause of many mine disasters.
In nature, methane is produced by the anaerobic bacterial decomposition of vegetable matter under water . Wetlands are the major natural source of methane produced in this way. Other important natural sources of methane include termites , volcanoes, vents in the ocean floor, and methane hydrate deposits that occur along continental margins and beneath Antarctic ice and Arctic permafrost. Methane also is the chief constituent of natural gas, which contains from 50 to 90 percent methane , and occurs as a component of firedamp along coal seams.
Why Different Elements And Compounds Have Different Melting And Boiling Points
There are many reasons to effect for melting and boiling points of elements and compounds. One or several of things may effect to melting and boiling points.
Don’t Miss: Why Am I Having Heavy Periods With Blood Clots
Thermal Conductivity Of Nonmetals
For nonmetallic solids, k is determined primarily by kph, which increases as the frequency of interactions between the atoms and the lattice decreases. In fact, lattice thermal conduction is the dominant thermal conduction mechanism in nonmetals, if not the only one. In solids, atoms vibrate about their equilibrium positions . The vibrations of atoms are not independent of each other, but are rather strongly coupled with neighboring atoms. The regularity of the lattice arrangement has an important effect on kph, with crystalline materials like quartz having a higher thermal conductivity than amorphous materials like glass. At sufficiently high temperatures kph 1/T.
The quanta of the crystal vibrational field are referred to as phonons. A phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, like solids and some liquids. Phonons play a major role in many of the physical properties of condensed matter, like thermal conductivity and electrical conductivity. In fact, for crystalline, nonmetallic solids such as diamond, kph can be quite large, exceeding values of k associated with good conductors, such as aluminum. In particular, diamond has the highest hardness and thermal conductivity of any bulk material.
Liquid Methane Rocket Fuel
In a highly refined form, liquid methane is used as a rocket fuel.
While investigations of methane use have existed for decades, no production methane engines have yet been used on orbital spaceflights. This is changing, and liquid methane has recently been selected for the active development of a variety of bipropellant rocket engines.
Since the 1990s, a number of Russian rockets have been proposed to use liquid methane. One 1990s Russian engine proposal was the RD-192, a methane/LOX variant of the RD-191.
In 2005, US companies, Orbitech and XCOR Aerospace, developed a demonstration liquid oxygen/liquid methane rocket engine and a larger 7,500 pounds-force -thrust engine in 2007 for potential use as the CEV lunar return engine, before the CEV program was later cancelled.
In October 2013, the China Aerospace Science and Technology Corporation, a state-owned contractor for the Chinese space program, announced that it had completed a first ignition test on a new LOX methane rocket engine. No engine size was provided.
it is abundant in many parts of the solar system and it could potentially be harvested on the surface of another solar-system body , providing fuel for a return journey.
SpaceNews is reporting in early 2015 that the French space agency CNES is working with Germany and a few other governments and will propose a LOX/methane engine on a reusable launch vehicle by mid-2015, with flight testing unlikely before approximately 2026.
Read Also: Can Period Panties Be Used For Incontinence
Can Methane Be Extracted From The Atmosphere
The bacteria can then be used to extract methane from the air. Because of its intense effectiveness as a greenhouse gas, Boucher and Folberth claim that the elimination of methane may be financially competitive with carbon capture, even if the technologies themselves are more expensive.
You can learn more about the different properties, applications, the structure of CH4 and other chemical compounds from the expert faculties at BYJUS Indias largest education company. Register now!
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Global Trends In Methane Levels
Long term atmospheric measurements of methane by NOAA show that the build up of methane leveled off during the decade prior to 2006, after nearly tripling since pre-industrial times. Although scientists have yet to determine what caused this reduction in the rate of accumulation of atmospheric methane, it appears it could be due to reduced industrial emissions and drought in wetland areas.
Exceptions to this drop in growth rate occurred in 1991 and 1998 when growth rates increased suddenly to 1415 nmol/mol per year for those years, nearly double the growth rates of the years before.
The 1991 spike is understood to be due to the volcanic eruption of Mt. Pinatubo in June of that year. Volcanoes affect atmospheric methane emissions when they erupt, releasing ash and sulfur dioxide into the air. As a result, photochemistry of plants is affected and the removal of methane via the tropospheric hydroxyl radical is reduced. However, growth rates quickly fell due to lower temperatures and global reduction in rainfall.
The cause of the 1998 spike is unresolved, but scientists are currently attributing it to a combination of increased wetland and rice field emissions as well as an increased amount of biomass burning. 1998 was also the warmest year since surface temperatures were first recorded, suggesting that anomalously high temperatures can induce elevated methane emission.
Don’t Miss: How To Have Sex On Period
